Background: Relapsed/refractory (R/R) DLBCL constitutes 40% of all DLBCL cases. Coventional salvage regimens, such as R-ICE, R-GDP, and R-DHAP, have an overall response rate (ORR) of 60% and a complete response rate (CR) of 30-40%. For patients who have achieved complete metabolic remission as determined by PET-CT evaluation after salvage therapy, undergoing autologous transplantation is crucial in order to achieve durable long-term survival. The outcomes of these R/R DLBCL remain to be improved.
Aims: This prospective study aims to investigate whether adding novel agents to R-ICE (R-ICE-X) based on genetic subtypes could improve clinical efficacy in R/R DLBCL. This trial is registered at clinicaltrials.gov (NCT05348213).
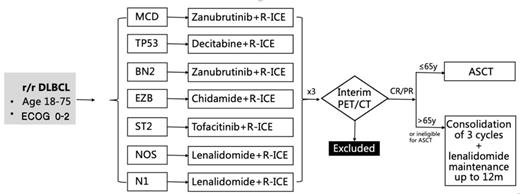
Method:In this study, patients with R/R DLBCL aged 18 -75 years were enrolled. All patients were assigned and stratified by genetic subtype and received different targeted agents combined with R-ICE. The efficacy was evaluated after 3 courses of R-ICE-X (every 21 days). Patients with CR/PR were subsequently treated with autologous hematopoietic stem cell transplantation or 3 courses of R-ICE-X consolidation and lenalidomide maintenance for up to 12 months. See the detail in the figure. The primary endpoint was the overall response rate (ORR), and the secondary endpoints were the 2-year progression-free survival (PFS) rate, 2-year overall survival (OS) rate, and safety evaluation.
Results: At the time of data cut-off, a total of 66 patients were enrolled, with a median age of 61(20-75) years. In the patients with R-ICE-zanubrutinib group (n=28), the ORR was 84.0%, with 15 patients (15/25, 60.0%) achieved CR and 6 patients (6/25, 24.0%) achieved PR. The 1-year PFS rate and OS rate were 68.2% and 90.5%, respectively. In the patients with lenalidomide combined with R-ICE (n=28), 16 patients (16/26, 61.5%) achieved CR, 4 patients (4/26, 15.4%) achieved PR, and the ORR was 76.9%. The 1-year PFS and OS rates were 78.5% and 100.0%, respectively. In the patients with the R-ICE-decitabine group (n=7), the ORR was 66.7%, with 4 patients (4/6, 66.7%) achieving CR. The 1-year PFS rate and OS rate were 50.0% and 60.0%, respectively. The number of other groups needs to be further updated. Among 66 patients, 17 patients had autologous transplantation (ASCT) and 1-year PFS and OS of patients with ASCT were both 87.1%. The number of other groups will be further updated.
Conclusion: Novel targeted agents in combination with R-ICE (R-ICE-X) regimen was well tolerated and efficacy in R/R DLBCL patients, meanwhile, it could be a promising bridging regimen for ASCT. The study is ongoing and further results will be continuously released.
Disclosures
No relevant conflicts of interest to declare.